Toluene is the common name of methylbenzene. Both are nonpolar molecules.
Difference Between Alkanes And Alkenes Definition Nomenclature Properties And Reactions
The general formula is CnH2n which is two hydrogen atoms less than the corresponding alkane.
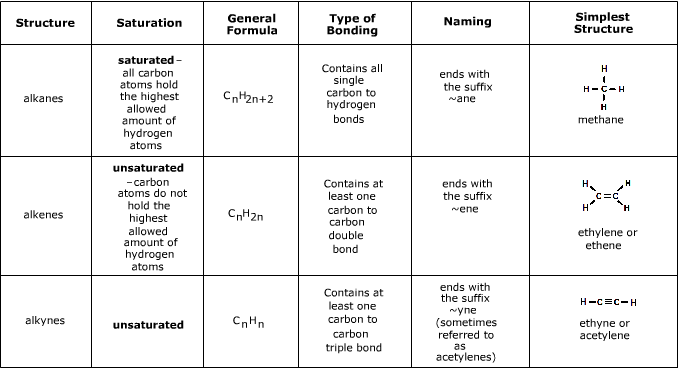
. Collectively they are called unsaturated hydrocarbons which are defined as hydrocarbons having one or more multiple double or triple bonds between carbon atoms. In the case of alkenes and alkynes hydrocarbon chain with the double and triple bond. Below are the differences between the four stated as a list.
The longest hydrocarbon chain is selected and is termed as parent chain in case of alkanes. Alkenes are hydrocarbons that contain one or more double bonds while alkynes contain one or more triple bonds. Organic chemistry is the study of compounds which contain carbon.
By definition alkenes are hydrocarbons with one or more carboncarbon double bonds R 2 CCR 2 while alkynes are hydrocarbons with one or more carbon-carbon triple bonds RCCR. Carbon has a valency of 4. A further difference between alkenes and alkynes is that the alkenes have no acidic hydrogen.
One of a set of the isomers of a compound that exhibits stereoisomerism. General formula for alkenes in the case of a non-cyclic compound is CnH2n. Thus called as saturated hydrocarbons They are less reactive in nature as the carbons bonds are stableThey are also called paraffins They are simplest of the hydrocarbons which have no functional groups attached to the carbon atoms.
Draw the distinguishing structural feature for the following. Alkenes are hydrocarbons compounds containing only C and H that have one or more CC double bonds two C atoms are linked by 4 shared electrons. It has the potential to react with bromine.
3Alkanes are the most stable hydrocarbons as the carbon. Similarities Between Alkanes and Alkenes Alkanes and alkenes are hydrocarbons. How do you remember the differences between alkanes and alkenes.
Alkenes are unsaturated hydrocarbons because they have a double C-C bond. With the same number of carbons the alkane should have a higher boiling point. Alkanes are saturated hydrocarbons with the general molecular formula of C n H 2n2 and alkenes are said to be an unsaturated hydrocarbon group since they contain.
Here are the names and structures of five alkanes. See full answer below. Between them by a straight line.
Toluene is classified as an aromatic compound. 1Alkanes are saturated hydrocarbons. One important property of alkynes is the acidic nature of the hydrogen attached to the triple bonded carbon because hydrogen is attached to sp hybridised carbon.
Alkenes are hydrocarbons with one or more double bonds. They undergo addition and oxidation reactions readily. Explain the difference between saturated and unsaturated hydrocarbons.
However theres something else in play here. Alkynes are hydrocarbons with one or more triple bonds. Each alkene has 2 fewer electrons than the alkane with the same number of carbons.
What is the difference between alkanes alkenes and alkynes. The atoms in alkanes and alkenes are bonded to each other through covalent bonds. The key difference between alkenes and alkynes is that the alkenes have carbon-carbon double bonds whereas alkynes have carbon-carbon triple bonds.
That means every carbon atom bonds to four other atoms. Alkanes alkenes alkynes aromatics 3. As explained since there is a bigger volume to an alkane than its corresponding alkyne ie.
Example of an Alkene Eg. Since the CCCC functional group has two π bonds alkynes typically react even more readily and react with twice as much reagent in addition reactions. Rules underlying IUPAC nomenclature of alkanes alkenes and alkynes are discussed below.
Therefore both are insoluble. Alkynes are unsaturated carbon that shares a double bond at the carbon site. Alkanes have single bonds between carbon atoms.
The main difference between alkenes and alkynes is the number of carbon atoms in each type of molecule alkenes have two while alkynes have three. 2General formula for alkanes is CnH2n2. Carbon is special because it can form covalent bonds with itself.
They have one or more triple bonds between the carbon atoms. 1 Answer anor277 Jun 7 2018 These differ by their degree of unsaturation. Alkane- They are hydrocarbons which are joined by single bonds only.
Draw the structure of toluene CH. Small alkane molecules and small alkene molecules are gases at room temperature. Alkanes have no.
Alkenes have the pi bond between the carbon atoms and during a lot of reactions the pi bond ruptures in order to form a single bond thus they are more reactive than alkanes but relatively stable as compared to alkynes. In the case of alkenes double bond linkages are seen and in alkynes triple bond linkages are present. Alkenes have double bonds between carbon atoms.
Alkenes undergo self addition in which alkene molecules join together to form long chains called polymers. Moreover Double bond carbons are sp 2 hybridized in alkenes and triple bond carbons are sp hybridized in alkynes. An unsaturated hydrocarbon containing at least one carboncarbon triple bond between two carbon atoms.
Alkanes alkanes have only single bonds between the carbons while alkenes contain at least 1 carbon-carbon double bond and alkynes have at least 1. 10 Differences between Alkenes and Alkynes. Common names are names given to many compounds but they may mislead the uninitiated.
Alkanes are completely saturated compounds. Chemically the alkynes are similar to the alkenes. Meaning a single bond between the carbon atoms.
Alkanes and Alkenes are two types of hydrocarbon families which contain carbon and hydrogen in their molecular structure. The Difference Between Alkanes Alkenes and Alkynes Common and Scientific Names. The key difference between Alkanes and Alkenes is their chemical structure.
Up to 24 cash back Alkenes Alkenes have a double bond. Alkynes Alkynes are also unsaturated hydrocarbons. Alkynes are unsaturated carbon that shares a triple bond at the carbon site.
Alkanes Alkenes and Alkynes. Alkanes are organic compounds that consist entirely of a single-bonded carbon and hydrogen atoms and lack any other functional groups. Alkynes have a TRIPLE bond.
Alkynes are hydrocarbons compounds containing only C and H that have one or more triple bonds two C atoms are. May 10 2016 May 11 2016 mrshakeelchemistry. Notice that the molecular models on the right show that the bonds are not really at angles of 90.
Organic Chemistry Ways to Draw and Represent Molecules Condensed Structure. They are more reactive than alkanes and alkynes due to the presence of two π bonds.

Differences Between Alkane Alkene And Alkyne Youtube
What Is The Difference Between The Alkanes And The Alkenes Quora
0 Comments